Metal Properties: Conductivity

In previous installments of our series on Metal Properties, we’ve discussed characteristics that set metals apart from one another, as well as those that distinguish them from other nonmetallic elements. Conductivity represents an array of essential features that go to the very heart of what defines a metal. In this article we will be asking: what are the factors affecting the conductivity of metals? What are the most thermally and electrically conductive metals, and why does conductivity matter for manufacturers?
All materials possess some degree of conductivity. One of the main characteristics of metals is their ability to conduct heat and electricity, so metals are all relatively conductive compared to non-metals. However, even within metals you’ll find a broad range in levels of conductivity. A sound knowledge of where various metals fall on the spectrum helps manufacturers choose the right alloy for each product.
What Is the Definition of Conductivity?
Physics defines five different types of conductivity: ionic, hydraulic, acoustical, thermal, and electrical. Most manufacturers are primarily concerned with the last two: electrical and thermal conductivity.
Electrical conductivity is a measure of how efficiently a material carries a unit of electric potential (also known as a charge). You can think of it as how easily a material allows an electric charge to pass through it without slowing it down. While this charge is passing through a material, we can observe distinct aspects of this electrical action and measure them independently. This enables us to achieve a more complete understanding of a workpiece’s conductivity.
- The difference in potential energy between two specific points is measured in volts (V)
- The actual amount of energy transported over a given time interval is measured in coulombs (C)
- And how efficiently a specific piece of material (with mass, length, and width) carries electric current is measured in siemens (S)
Thermal conductivity, on the other hand, is a measure of the rate at which heat is transferred through a material. Thermal conductivity is governed by rules under the second law of thermodynamics: namely, that heat will flow from a hot point toward a cold point until the temperature difference between them is equalized. Thermal conductivity is measured in watts (W).
There is a lot of overlap between thermal and electrical conductivity because the atomic building blocks that make metals so very special are the same blocks that set the stage for their terrific conductivity.
Conductivity vs Resistivity
Resistivity is the reciprocal of conductivity, meaning they are two sides of the same coin. If your material isn’t conducting, it’s resisting. If it isn’t resisting, it’s conducting. Whether you’re measuring resistivity or conductivity is a matter of application. If you’re building insulators, it’s resistivity you’re measuring. If you’re extruding copper wire, you want to know its conductivity.
Understanding Electrical Conductivity on a Molecular Level
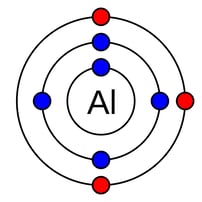 Even if you haven’t taken a chemistry class in a while, you’ll probably remember the two most common types of chemical bonds: ionic and covalent. There are, in fact, more than two types of chemical bonds, and metals have their very own way of atomic assembly. A defining property of metals is that some of their electrons are very weakly attached to the nucleus. They are known as valence electrons and occupy the outermost orbit of the atom.
Even if you haven’t taken a chemistry class in a while, you’ll probably remember the two most common types of chemical bonds: ionic and covalent. There are, in fact, more than two types of chemical bonds, and metals have their very own way of atomic assembly. A defining property of metals is that some of their electrons are very weakly attached to the nucleus. They are known as valence electrons and occupy the outermost orbit of the atom.
Right: diagram of Aluminum molecule with valence electrons indicated in red.
When metal atoms start coming together, those valence electrons are so loosely attached to their ‘home’ atom that they start to float freely and move around the entire metal. They become what are known as ‘delocalized’ electrons. And there are lots and lots of them – so much that it becomes impossible to know which electrons belong with which atoms. The result is something like the figure below – notice how the electrons are detached from their home nucleus, floating in what scientists call an ‘electron sea’:
What enables valence electrons in metals to break away so easily? When valence electrons get excited, they can jump from the orbit of their home atom into the free-fly zone of the metal’s overall structure. In scientific jargon, we say that a valence electron gets ‘promoted’ to a conduction electron. This happens very easily in metallic elements simply because the energy needed to promote an atom is very small relative to non-metallic elements. The term for this type of energy is called ionization energy.
With the electrons now broken away from their home atom, the combination of the negatively charge delocalized electrons and the now positively charged nuclei creates electrostatic force. And it is this force that holds the atoms together. In fact, electrostatic force is what makes metallic bonding possible.
So, what happens when we send a charge through the metal? The delocalized electrons become kinetic and carry the charge at remarkable speed. And because they can travel anywhere, and number in the billions or even trillions, there’s no lack of available electrons to pass on the electric message.
Learn more about metal properties with our free 47-page ebook. Click here to download.
Understanding Thermal Conductivity on a Molecular Level
Thermal conductivity and electrical conductivity are closely related, but they function in different ways. To help you visualize this difference, imagine standing with your hand on one end of a 20ft copper wire about an inch in diameter. Your friend on the other end sends an electric current through it (let’s hope it’s a small one). The current reaches you almost instantaneously. In round two of this experiment, your friend heats the wire. Even if the heat is significant, you probably have several seconds before your side of the metal warms beyond a tolerable level. Despite the difference in speed, delocalized electrons are behind both types of conductivity.
Remember that with electrical conduction, the atoms are zooming up and down the metal at remarkable speed. When a metal is heated, however, the valence electrons don’t speed around; they shake and vibrate. And once they’re vibrating with enough intensity, they start to bump into other electrons around them, transferring some of their heat energy to their neighbors. This results in a slower transfer of energy compared to electrical conduction.
You might be wondering: are thermal and electrical conductivity in metals complementary, or can they be at odds with each other? Can a metal have high electrical conductivity and low thermal conductivity? You’re asking the right questions, but there aren't necessarily simple answers. Among metals, thermal conductivity and electrical conductivity are very strongly correlated (see table below). But within any given metal, if you crank up the heat and the molecules start vibrating enough, their wobbly behavior will worsen electrical conductivity. Why? Because all the vibrations create lots of random particle collisions, which weakens the forward flow (current) of electrons.
Which Metals Have the Highest Conductivity? What are their applications?
Metals are great conductors because of their atomic structure, as we’ve just explored. Metals are also widely available, making them economical raw materials with which to manufacture commercially useful conductors. When it comes to commercial and industrial applications, the key is to find the right material at the right price without jeopardizing performance.
A good example of applied thermal conductivity is a heat sink. Heat sinks are the little fans inside your computer that draw heat from electrical components to cool them and they’re often made from aluminum. Although aluminum’s thermal conductivity is much lower than that of copper, its conductive properties are adequate for this application, and refined aluminum is significantly cheaper than refined copper.
With electrical conductivity, copper is king. The seafloor is lined with copper wires to move data from one continent to another. Your house is lined with copper wiring, without which you’d have no electricity. But aluminum is also widely used for electrification. One example is for long-distance power lines – because aluminum is lighter, it keeps the installations from collapsing under their own weight, as they could with copper.
How do we know which metals are the most conductive? It comes back to the concept of ionization energy that we discussed above: the metals topping the conductivity tables require the smallest amount of ionization energy. In the hierarchy of most conductive metals, silver leads the charge (pun intended). But as silver is exponentially more expensive than copper and aluminum, it’s only used in very specific cases where extreme conductivity is needed.
Finally, you might be asking: is conductivity a property limited to metals? No, conductivity is not limited to metals, or even to solids. We can also measure the conductivity (or resistivity) of liquids, gases, and plasma. In fact, the absolute best electrical conductors are called superconductors, and they’re usually composed of various nonmetallic elements. And THE greatest thermal conductor out there is a diamond. Interestingly, however, diamonds have almost zero electrical conductivity! (a story for another day).
Here is a chart with conductivity measurements of some of the most widely used metals and alloys:
Metal |
Electrical Conductivity (10^6 siemens/m) |
Electrical Resistivity (10^-8 ohms/m) |
Thermal Conductivity
|
| Silver | 62.1 | 1.6 | 420 |
| Copper | 58.7 | 1.7 | 386 |
| Gold | 44.2 | 2.3 | 317 |
| Aluminum | 36.9 | 2.7 | 237 |
| Molybdenum | 18.7 | 5.34 | 138 |
| Zinc | 16.6 | 6.0 | 116 |
| Brass | 15.9 | 6.3 | 150 |
| Nickel | 14.3 | 7.0 | 91 |
| Steel | 10.1 | 9.9 | 80 |
| Bronze 67Cu33Sn | 7.4 | 13.5 | 85 |
| Carbon steel | 5.9 | 16.9 | 54 |
| Stainless steel 316L EN1.4404 | 1.32 | 76.0 | 15 |
How Conductivity Helps Manufacturers Choose the Right Alloy
Knowing the physical rules of conductivity helps manufacturing professionals choose the right material for their final products. Some questions to bear in mind are:
- How much electrical energy will be passing through the part?
- Is thermal regulation an important component of the part?
- Will the part require rapid cooling or heating?
- Will the part be welded?
For example, the part may be used in humid environments where corrosion resistance and low thermal conductivity are desirable properties. You may require strong conductivity because your part is involved in electrical installations. If you are working in an environment where heat energy is frequently fluctuating, you may want a metal with high thermal resistance. Parts used in the transfer of heat, like induction furnaces, have some components with high thermal conductivity, and others with low thermal conductivity.
Conductivity is essential in welding applications. Arc welding involves mobilizing an electric current to heat a metal enough to melt it with another adjoining metal. If your material isn’t sufficiently conductive, you won’t generate enough heat to melt the metals together.
At the Eagle Group we take metal properties seriously, and conductivity is no exception. Our experienced staff is available to advise you on optimal alloys for your products, and our casting and machining facilities are ready to work with a broad range of alloys. Whether conductive or resistive, we have what it takes to deliver quality parts for any application.
To learn more about metal properties, check out our 47-page ebook, Metal Properties and Their Applications in Metalcasting and CNC Machining. Click below to download:
Tags: Metal Properties, Physical Properties, Conductivity

Written by Jason Bergman
Jason Bergman is Senior Quality Engineer and Metallurgist at Eagle Alloy. He has been with the company since 2013.




.jpeg?width=525&name=Electron_Sea_(Plasma).jpeg)

